Registering Tissue Banks
Tissue Bank Registration
1) Download and complete the Tissue Bank Application Form*
2) Seek endorsement from your Head of Department (HOD)
or equivalent
3) Seek endorsement from your Institutional Tissue Bank Committee (ITBC)* or
equivalent
4) Submit the completed form to the NHG TCC Secretariat
(NHGTCCSecretariat@nhg.com.sg)
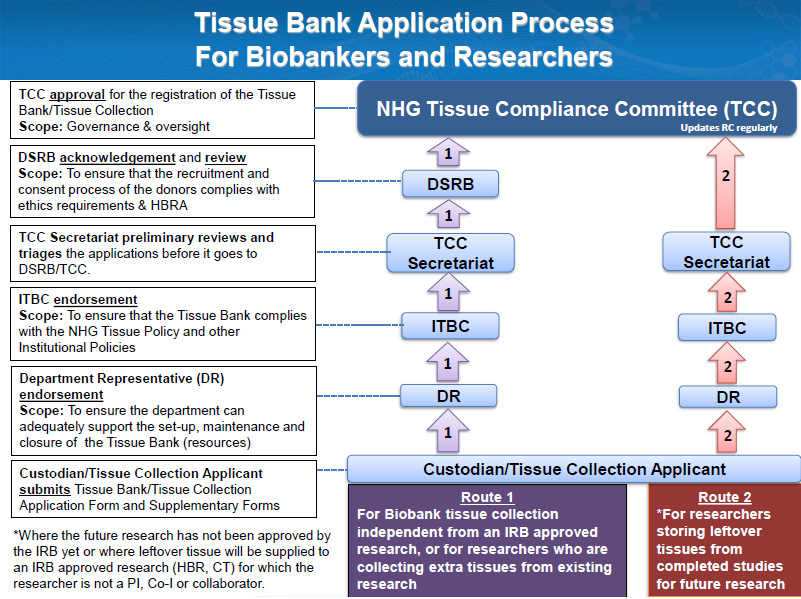
Fig. 1 – Tissue Bank Application Process in NHG
For collection of human tissue that is for the sole purpose of bio-banking and is not linked to any IRB-approved research, custodians must also complete the NHG Tissue Collection Form*.
Tissue Review Forms and Templates
-
Tissue Bank Application Form*
This form is submitted to the TCC to register a Tissue bank
-
Tissue Collection Application Form*
This form must be submitted to the TCC if a NHG staff intends to actively recruit donors independent of an IRB approved research for the sole purpose of collecting tissue to facilitate future research.
-
Declaration of Compliance to HBRA on Handling of Legacy Human Biological Material (HBM)*
This form is required for NHG staff who are in possession of Legacy HBM and intends to store or use them for future research from 01 November 2019
-
Other Supplementary Forms*
For reporting of Non-compliances, Amendments, Continuing Reviews, Serious Adverse Events (SAEs), & Untoward Occurrences
-
NHG Tissue Bank Policy, PCT SOPs* & Templates*
Policy, guidelines and templates developed by NHG Tissue Compliance Committee to provide detailed procedures on conducting tissue banking activities in accordance with applicable guidelines and regulations (e.g. HBRA).
Other Resources
-
Requirements for Appropriate Consent - Exemption Regulations
-
MOH Guidance on Prohibition Against Commercial Trading of Human Tissue under HBRA (February 2017)
* These documents are strictly for internal circulation among NHG Staff and Authorized personnel only. Access to these files is Restricted (NHG Intranet access required).

