Overview of Minimum Training Requirements
Introduction to Minimum Training Requirements
With Singapore’s push to become a regional hub for biomedical research and the increasing number of clinical trials, there is a growing need to ensure that all key investigators, such as the Principal Investigators, Site Principal Investigators, Co-Investigators and other staff who are involved in research activities have basic research ethics training. The intent of having minimum training requirements is for Principal Investigators and the research community to appreciate and apply the underlying ethical principles to their day-to-day research practice.
Minimum Training Requirements for Staff from NHG Health and Partner Institutions
There are 3 main types of training courses available:
-
Collaborative Institutional Training Initiative’s (CITI Program) Investigator’s Course
-
Financial Conflicts of Interest (FCOI) Course (sub-component of the CITI Program)
-
Good Clinical Practice (GCP) Course (based on ICH GCP, Singapore regulations and local IRB requirements)
The minimum training requirements for Staff from NHG Health and partner institution differ according to their study roles. The Table below summarizes the minimum training requirements.
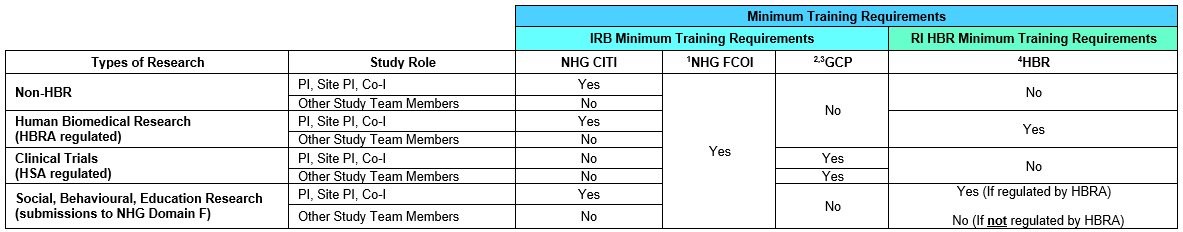
1 All investigators, study team members and external institution staff involved in the design, conduct or reporting of research in institutions under the oversight of NHG Health DSRB are required to complete the Financial Conflict of Interest (FCOI) Course.
2 The DSRB will recognise generic GCP courses and training as acceptable minimum training requirement. PIs, Site-PIs Co-Is and other study team members may complete either local or overseas GCP training programmes, e.g., CITI ICH E6 (R3) GCP Training Course, general GCP training provided by sponsor companies. PIs, Site PIs and Co-Is who have completed the ICH GCP course may use it as an alternative minimum requirement for biomedical research studies only. Refer to the Good Clinical Practice (GCP) Training for more information.
3 Mandatory for Study Team Members (STM) conducting significant trial related tasks (informed consent, eligibility assessment, investigational product management, key efficacy, and safety assessments, etc.) to complete GCP training before study involvement. Refer to the Good Clinical Practice (GCP) Training for more information.
4 HBR Minimum Training to be completed by all NHG Health Investigators and study team members involved in the design, conduct or reporting of HBR studies:
-
PI, Site PI, Co-I : Before new HBR IRB submissions and amendments for HBR studies in ECOS
-
CRCs, RAs, other study team members: Prior to the commencement of their study involvement.
Note: For Investigators and study team members from NHG Health partner institutions, please refer to the respective Research Institution (RI) HBR minimum training requirements.
The SingHealth CIRB requirements will apply to staff from SingHealth and partner institutions who are involved in cross-cluster studies that are submitted to DSRB for review. Refer to the CIRB website for more details.
Contact Information
For any enquiries, please contact the NHG Health Minimum Ethics Training Secretariat:
Email: nhggroup.min.ethics.training@nhghealth.com.sg
For any HBR enquiries, please contact the NHG Health Research Course Admin (HBR only):
Email: nhggroup.research.courseadmin@nhghealth.com.sg
DID: 6471 3266
Office of Human Research Protection Programme (OHRPP)
NHG Health Group Research & Innovation
To contact your Institutions’ Minimum Training Secretariat (MTS) or NHG Health Research Course Admin (for HBR ERC), please refer to the following:
|
Institution |
Institutional MTS Contact Information |
|---|---|
|
Geriatric Education & Research Institute (GERI) |
Ms Qiu Shijia: qiu.shijia@geri.com.sg |
|
Institute of Mental Health (IMH) |
Ms Jenny Tay: Jenny.am.tay@nhghealth.com.sg Ms Jaclyn Ong: Jaclyn.yy.ong@nhghealth.com.sg |
|
Khoo Teck Puat Hospital (KTPH) |
Ms Vimala: vimala.sadaiyappan@nhghealth.com.sg |
|
NHG Health Polyclinics |
|
|
National Skin Centre (NSC) |
|
|
Tan Tock Seng Hospital (TTSH) |
|
|
Woodlands Health (WH) |
Ms Liang Shanying: shanying.liang@nhghealth.com.sg |
Updated: 17 Oct 2025

