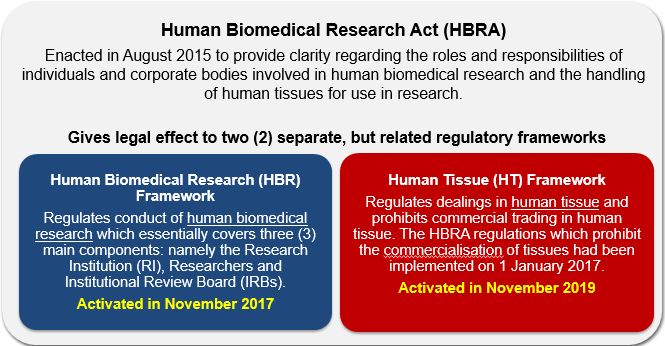
Human Biomedical Research Act (HBRA)
Information Pack for Researchers on the HBR Framework
The following materials provide researchers involved in the conduct of HBR activities a quick overview of the HBRA and its subsidiary legislations.
-
Section I: HBR Background & Framework
-
Section II: Studies excluded from HBRA
-
Section III: Restricted & Prohibited Research
-
Section IV: HBRA and its implications on Studies
HBR Resources
-
Human Biomedical Research (Requirements for Appropriate Consent – Exemption) Regulations 2019
-
Human Biomedical Research (Tissue Banking – Exemption) Regulations 2019
For more information, please refer to the MOH website.
HBR Case Studies
The NHG Health RI shares good practices and negative examples using short case studies on a regular basis to help researchers understand the what-to’s and how-to’s of HBRA.
Download the HBR Case Studies here (Restricted: NHG Health Intranet access required).
Restricted Human Biomedical Research (rHBR)
HBR involving human eggs/embryos or human-animal combination are restricted, and subject to tighter controls.
Restricted HBR requires obtaining approval from Institutional Review Board (IRB), Institutional Animal Care and Use Committee (IACUC) where applicable, and the Director of Medical Services, amongst others.
Download the rHBR information sheet here. (Restricted: NHG Health Intranet access required)

