Applying for Ethics Approval
Application Process
All research applications must be submitted to the DSRB via the Ethics and Compliance Online System (ECOS).
Once the Principal Investigator submits an application via ECOS, it will
be automatically routed to the Department Representative (DR) and subsequently
the Institution Representative (IR) for endorsement. The DSRB will receive
the application only after both the DR and IR have endorsed it.
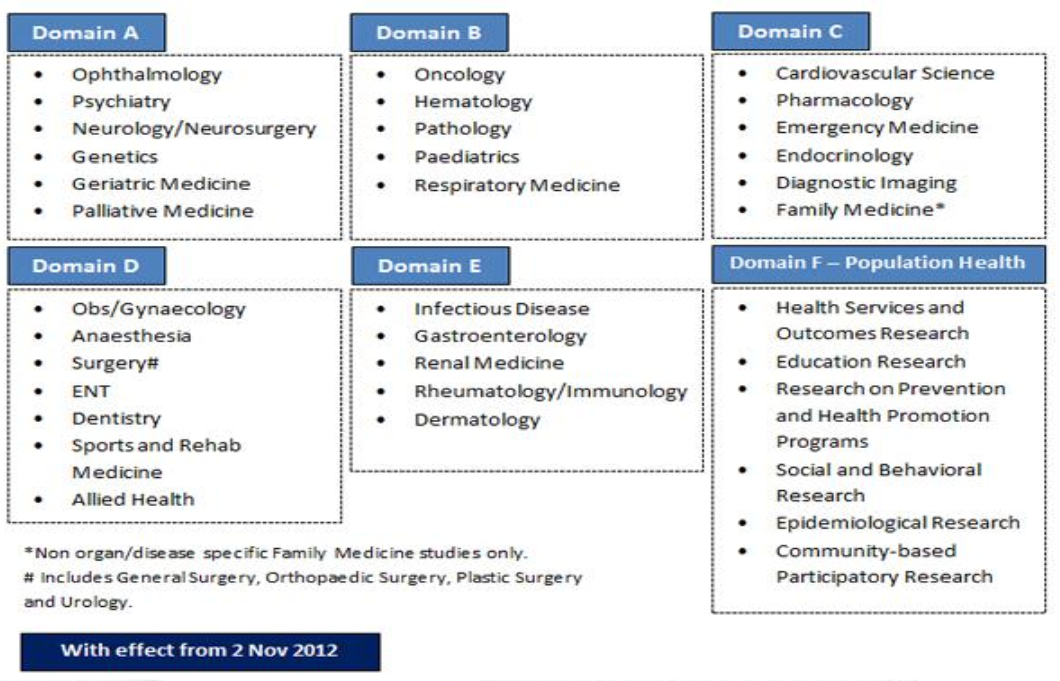
The Principal Investigator should select the most appropriate DSRB domain
to review their study. The DSRB may re-assign your application to another
Domain upon preliminary review of your application.
Review Category
Based on the level of risk in which research participants will be exposed
to, all research studies submitted will be classified under one of the
following review categories:
Exempt Review
Research studies that involve anonymous surveys and questionnaires, collection or study of anonymous existing data or tissue specimens, where data/tissue are either publicly available or subjects cannot be identified, or public benefit programmes.
Expedited Review
Research studies that involve collection of data or biological samples via non-invasive procedures, medical case-notes review, surveys or interviews with identifiers.
Full Board Review
Research studies that do not qualify for exempt or expedited review will be reviewed under full board review. Such studies may include research studies that involve the study of the safety and efficacy of a medicinal product, medical device, or research study that involve invasive procedures.
Studies that fall into the exempt and expedited review categories will
be reviewed by the Domain Chairperson at the weekly chair meeting.
Studies that require full board review will be reviewed at the monthly
convened meeting at which a quorum is present.
All new applications accepted by the DSRB will be reviewed within thirty
calendar days. However, the time frame from submission to approval will
vary depending on factors such as the completeness of the application,
complexity of the study, the response of the Investigator to DSRB's queries
and availability of a meeting quorum.
DSRB Full Board Review Meeting Schedule
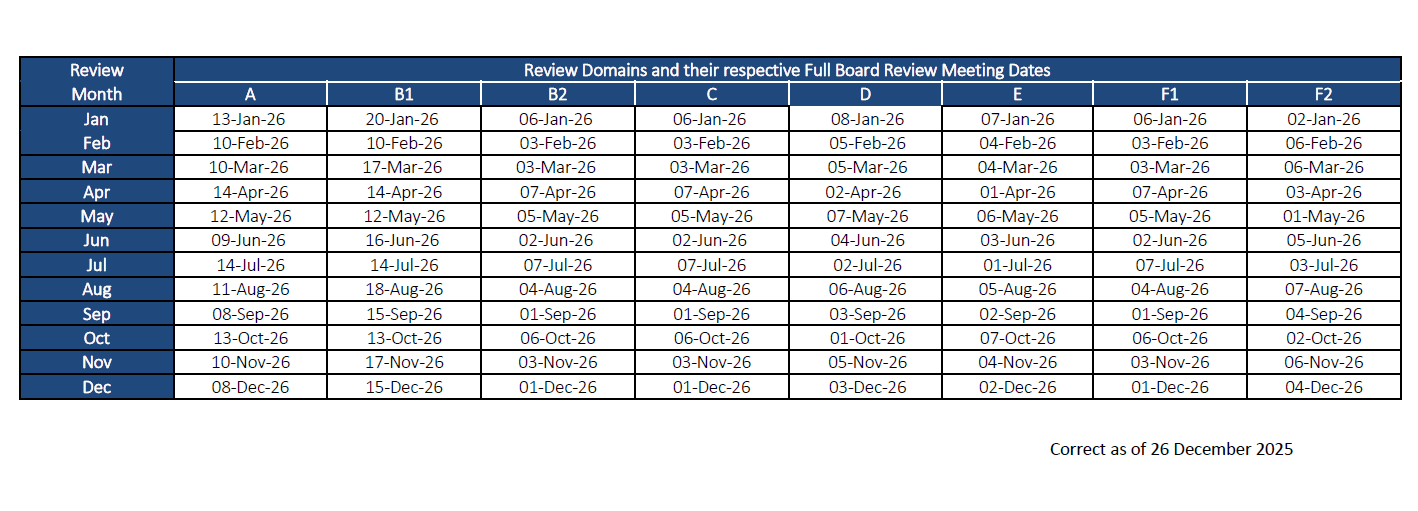
Please note that Full Board meeting dates may change without prior notice.
Kindly note that the DSRB submission deadline for Full Board studies is on the 15th day of the month or the next earliest working day if that day falls on a weekend.
This is with the exception of Domain B1 whereby the submission deadline for Full Board studies would be on the 1st working day of the month or the next earliest working day if it falls on a weekend.
Do note that applications should be submitted with sufficient lead time for the Department Representative and Institutional Representative to endorse prior to the submission deadline for the month.
Studies that fall into the Exempt and Expedited review categories will be reviewed by the Domain Chairperson at the weekly chair meeting.
Review Criteria
All research studies that intend to involve human subjects, use their
biological samples and/or data, must meet certain criteria before study
procedures can be initiated. The criteria are based on the principles of
autonomy, beneficence and justice as discussed in the Belmont Report.
In general, a research study must fulfill the following criteria:
-
Risks are minimized and are reasonable in relation to anticipated benefits.
-
Selection of subjects is equitable.
-
Informed consent will be sought, and appropriately documented.
-
Adequate provision for monitoring of data to ensure safety, protection of privacy of research participants and confidentiality of data collected.
-
Additional protection for vulnerable populations.
Resources: See the DSRB Review Requirements & Process Guide here.

